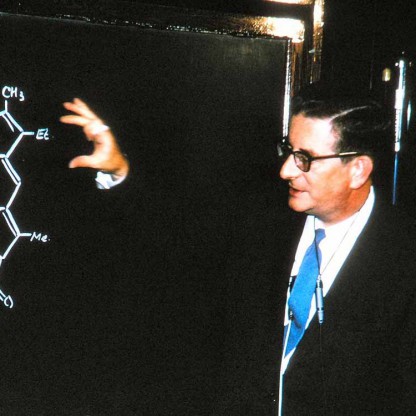
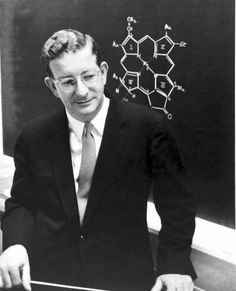
From a very early age, Woodward was attracted to and engaged in private study of chemistry while he attended a public primary school, and then Quincy High School, in Quincy, Massachusetts. By the time he entered high school, he had already managed to perform most of the experiments in Ludwig Gattermann's then widely used textbook of experimental organic chemistry. In 1928, Woodward contacted the Consul-General of the German consulate in Boston (Baron von Tippelskirch ), and through him, managed to obtain copies of a few original papers published in German journals. Later, in his Cope lecture, he recalled how he had been fascinated when, among these papers, he chanced upon Diels and Alder's original communication about the Diels–Alder reaction. Throughout his career, Woodward was to repeatedly and powerfully use and investigate this reaction, both in theoretical and experimental ways. In 1933, he entered the Massachusetts Institute of Technology (MIT), but neglected his formal studies badly enough to be excluded at the end of the 1934 fall term. MIT readmitted him in the 1935 fall term, and by 1936 he had received the Bachelor of Science degree. Only one year later, MIT awarded him the doctorate, when his classmates were still graduating with their bachelor's degrees. Woodward's doctoral work involved investigations related to the synthesis of the female sex hormone estrone. MIT required that graduate students have research advisors. Woodward's advisors were James Flack Norris and Avery Adrian Morton, although it is not clear whether he actually took any of their advice. After a short postdoctoral stint at the University of Illinois, he took a Junior Fellowship at Harvard University from 1937 to 1938, and remained at Harvard in various capacities for the rest of his life. In the 1960s, Woodward was named Donner Professor of Science, a title that freed him from teaching formal courses so that he could devote his entire time to research.